Functions of Regulatory Bodies in Healthcare
| ✅ Paper Type: Free Essay | ✅ Subject: Health And Social Care |
| ✅ Wordcount: 3237 words | ✅ Published: 23 Sep 2019 |
Part 1. Explain the functions and roles of the following:
Notified bodies
Notified bodies are an organisation managing the assessment and testing of products, and are more considered private bodies rather than government controlled (McDermott 2019). The European Commission produces a list of organisational bodies that serve to accomplish tasks associated with conformity assessment procedures before a product’s release on the market, especially during third party involvement. Manufacturers can avail of the assessment of compliance provided by the notified bodies in relation to public interest. Notified bodies must be informed by the relevant EU authorities of their range of power based on principles declared in Decision 768/2008/EC. Notified bodies can provide their service for any operator and on any territory, regardless of whether in the EU or not (Europa 2019).
Furthermore, they are required to act in a transparent, inclusive and non-biased manner, and must hire staff with the best skill range and experience for their position so that the conformity assessment can be carried out with the relevant laws in mind. Confidentiality is of utmost importance for data protection and as such the notified bodies must make necessary arrangements to uphold that. Adequate insurance beforehand is necessary so that any losses during professional activity are covered or else must be assured by the relevant EU authorities as backed up. The notified bodies always keep the notifying authority and any market surveillance authorities informed of every activity. As such, the notified body must be monitored to ensure certain practices and regulations are met. Accreditation organizations are in charge of checking their competence on a regular basis; generally using the EN ISO/IEC 17000 accreditation and standards series for helping with the monitoring of conformity to relevant legislations (Europa 2019).
Competent Authorities
A National Competent Authority is one that has legal ability to carry out certain pertained tasks, most commonly the monitoring of national regulations and statutes and ensuring compliance occurs (Europa 2019).
These policymaking agencies are responsible for the introduction of national regulations. Not unlike departments in government, the agencies also have appointed Ministers of Health. A large variety of advisory panels direct the authorities, and they are of a highly technical nature. As reflected in the below Table 1.1, roles and responsibilities consists of:
Table 1.1 Roles and responsibilities of National Competent Authorities (McDermott 2019).
|
|
|
|
The NCAs manage the authorization process for medicines not adhering to the centralised procedure in the EU. The European Medicines Agency closely cooperates with the national competent authorities of the Member States of the European Union (EU) and the European Economic Area (EEA) responsible for human medicines (Europa 2019).
NCAs manage the regulations for medicine in the EU, both human and veterinary. Their forces are combined in the Head of Medicines Agencies (HMA) forum. The European Commission and the EMA work together with the NCA leaders to warrant maximum collaboration and a highly functioning regulatory network for European medicines. Quarterly meetings occur in the HMA so as to discuss the best strategies to uphold the network and focus on areas such as developments in IT and information exchange so that the decentralised procedures and co-recognition are up to date (Europa 2019).
HPRA
The Health Products Regulatory Authority (HPRA) is an Irish-based authority initially founded as the Irish Medicines Board, which covered only drugs, but now also includes medical devices and products (McDermott 2019).
Their role is to regulate various health products, from medical devices to drugs and even cosmetics, for the benefit of public safety in relation to both human and veterinary practises. It is a state agency that assesses and controls health related produce created in Ireland or exported overseas using clinical and scientific expertise, with the aim of ensuring health products work as envisioned. The upgrade in name was due to an expansion of regulatory functions that is, extending to include practices such as clinical trials, medical devices, tissue and blood components, cosmetic products, animal welfare in relation to scientific trials, controlled drugs and organ donations (HPRA 2019).
Moreover, the HPRA produces company licenses for creating and distributing medicines on the market post close scrutiny of the quality and safety, along with inspecting those organizations for compliance with national legislation and standards. All health-related produce is constantly monitored so any arising issues are resolved immediately, and safety and quality information is always provided to ensure only the best practices occur. The agency is responsible for the regulation of any type of organizations dealing with medicine, including various distribution and wholesale companies, regulating the manufacturing process itself, other health product amenities and any notified bodies dealing with medical devices. The agency also provides licenses for the usage of animals in scientific or educational trials and inspects the process to warrant the application of the principle of 3R, that is, reduction, refinement and replacement (HPRA 2019).
EMA
The EMA is the European Medicines Agency, which is a European regulatory body for all pan-European companies. This agency relies on several scientific committees that are of paramount influence when it comes to the agency’s well-being, as they are the element responsible for decision-making and issuing advice in relation to medicines. A multitude of committees exist targeting varying sectors such as reflected in below Figure 1.1:

Figure 1.1 EMA committees, adopted from (Europa 2019).
Any pharmacological companies that produce new high-end medications for the EU must have their produce scientifically reviewed by the EMA. In 1995 the EMA was founded to guarantee supervision of medicines in Europe and the most efficient allocation of scientific resources. Members of the EMA are generally experts who operate in various fields such as scientific advisory groups, scientific committees, and working parties are part of national assessment teams who deal with medicine assessment. Members are generally selected based on their expertise in the field, with many being put forward by National Competent Authorities (NCAs) from states who are members (Europa 2019).
There is a rise of individuals who are proficient in healthcare or are patients themselves that are becoming involved in this process too. Thus, the EMA uses these expert opinions from its members to establish scientific guidelines, and as such are an accurate representation of current discussion and views about biomedical science developments. Any medicine developer can then use these guidelines to guarantee the highest quality of medicine and during the marketing authorization process when proposing an application. As well as, the EMA provides advice related to companies for the production of specific medicines. Thus, this is an extremely beneficial instrument for developing safe medicines of the highest quality and making them available to customers. The NCAs can also provide such advice (Europa 2019).
FDA
The Food and Drug Administration (FDA), which is the biggest regulatory authority on a global scale, is a US agency who is the main supervisor for food, devices, cosmetics and drugs in the country, as well as being a branch of the Department of Health and Human Services (HSS). It employs almost 10,000 professionals who do a large portion of the safeguarding of the US citizens health. The President, along with the Senate’s consent, is who chooses a Commissioner for the task of overseeing this agency.
Some of the tasks the FDA performs is developing regulatory policies, establishing guidelines for the FDC act, examining products and facilities for compliance with legislations, assessing and approving submissions of devices and such, before their market release, protection against false advertising in relation to health products, along with contributing to international goals concerned with global harmonisation (McDermott 2019).
The FDA contains nine Offices and Centers (FDA 2018).
The FDA acts on public health and safety and regulates basic produce like food and cosmetics, but also tackles radiation emitting products and everything related to tobacco consumption. In addition to that, the FDA is a major component of the US’ anti-terrorism development FDA (2018).
The FDA has a very broad regulatory authority over the key areas already mentioned, but it’s role can be very similar to what some other state bodies are already responsible for. Hence, it is not uncommon for consumers to be uncertain about what regulatory agency is relevant for their issue and whom to contact. Generally, the FDA is associated with monitoring traditional product classes and their appropriate subheadings (2018). Some major ones are reflected in below Table 1.2. This list is not exhaustive.
Table 1.2. FDA product classes and subcategories (FDA 2018).
|
Product Class |
Examples of Subheadings |
|
Foods |
Food additives, dietary supplements |
|
Drugs |
Prescription and non-prescription drugs |
|
Biologics |
Vaccines, genetic produce |
|
Medical devices |
Prosthetics, surgical implants |
|
Radiation emitting products |
Mercury vapour lamps, x-ray products, sunlamps |
|
Cosmetics |
Perfume, skin cleansers |
|
Veterinary produce |
Livestock and pet food, veterinary drugs |
|
Tobacco produce |
Cigarettes, cigars |
The FDA’s approach to accepting market release of products varies depending on the product. It is based on relative risk to consumer health and laws already in place by the FDA. Medical products must have their benefits outweigh their risks, as risks will always be present in every regulated product, and thus it is important these judgements are taken seriously. The only exception for a product to pose more risk than benefit is if it is potentially lifesaving.
Complex products like innovative drugs or prosthetics must be approved as reliable and safe beforehand, while cosmetics or dietary supplements can be marketed without approval beforehand. The FDA does no testing or development itself. Average time for drug review is around 6 months (FDA 2018).
The FDA produces information in relation to product recalls and updates the press and other agencies continuously (FDA 2017).
IMDRF
The International Medical Device Regulators Forum (IMDRF) was established in the October of 2011, but was envisioned earlier that year as a forum for the discussion of the synchronisation of medical device regulations. It was established by a selection of medical device regulation representatives from all over the globe, including the World Health Organisation, who volunteered to gather in Ottawa to discuss the development of this forum. The goal was to use the Global Harmonization Task Force on Medical Devices (GHTF) as the basis was their idea and work from that to develop a more synchronized system for international medical device regulation (IMDRF 2019).
Regulatory officials are placed on the IMDRF Management Committee for the supervision of their policies and strategies, along with managing membership and overall Forum activities. Additionally, this Committee also manages the Working Groups that use the expertise of a diverse range of stakeholders from fields such as academia, healthcare, patients/consumers and industry. Some of the Harmonization Initiatives already undertaken by IMDRF are in relation to working with affiliate organisations, that is, APEC LSIF Regulatory Harmonization Steering Committee, the Pan American Health Organization (PAHO), and The Asian Harmonization Working Party (AHWP), all overlooked by the Official Observers (IMDRF 2019).
A set number of Official Observers from WHO or similar regulatory authorities is delegated by the Management Committee through a common vote, based on who would deem most valuable to IMDRF at the time. Like Members, Official Observers are required to be highly familiar or informed about current IMDRF issues but do not partake in the process of decision making (IMDRF 2019).
Affiliate Organisations are very important for IMDRF goals. They are regional or international bodies sharing the same interest in upholding convergence of medical device regulatory practises, such as by the tactical spread of knowledge and resources for the aim of developing reliable and safe medical on a global scale, and as such IMDRF likes to establish really good working relations with them (IMDRF 2019).
Part 2: In relation to Regulations and Regulatory Strategy, Explain the goals, principles and aims of regulation
Public health protection is a prerogative of all regulations. As such, the key points to consider are the efficiency, purpose, risk vs benefits, quality and above all, safety.
In relation to safety, the product should ideally cause no harm, and the regulations that are to be implemented should always take relevant measures to ensure product safety (Tobin 2009).
In relation to medical devices, the safety factor is considered as the most critical. A syringe aimed for blood collection can serve as a good example. A blunt needle or malfunctioning patient monitor can damage patient health and result in a critical situation (WHO 2003).
However, safety can never be absolute as no device is ever risk-free, with some flaws not exposed until much later. A prime example for this would be implants or prosthetics which spend considerable amount of time in the human body, only to have a serious unpredicted defect years later. Devices can fail based on factors due to unique conditions in a patient, or in an unpredictable manner not related to anything. Risk assessment is a process where one identifies the chances of a device failing with hazardous side effects (WHO 2003).
A clinically effective device is one which produces the desired effect (WHO 2003). Thus, one must also establish the intended usage for the product (Tobin 2009). If the product is intended for pain relief, it is necessary for the developer to have clinical results backing it up along with causing actual pain relief. Effectiveness is the same as efficacy for current clinical levels of a controlled environment.
When talking about efficacy, medical devices must not compromise on the well-being of patients or any other persons involved. Thus, it is of utmost importance the risk vs benefits are always calculated, with the priorities always placed on the benefit aspect. This is referred to as risk management. There exists a protocol by the International Organization for Standardization (ISO) for assistance with such. It provides a framework for manufacturers for the evaluation and management of risk in relation to the medical device industry (WHO 2003).
Solid evidence such as input from patients during the risk/benefit assessment must be provided beforehand so that all decisions can be made in the most rational and informed manner (FDA 2016).
Quality is another aspect to consider. Safety and fitness for purpose are the two major characteristics that one would consider for a product to be seen as of high quality. However, there are other major parameters of a quality product and services such as reliability and consistency. Quality systems serve the purpose to achieve appropriate level of the described parameters by many companies. Businesses in other industries can use quality systems on voluntary basis. This is not the case for producers of potential high-risk medical devices and drugs. Such businesses are legally obliged to have appropriate quality systems in place. Moreover, there are regulations that define specifics for the quality systems to be applied within such businesses (Tobin 2009).
In its own turn, implementation of quality management requires resources, procedures, organizational structure, responsibilities and processes that are defined by quality system. Requirements of quality systems can influence all stages of medical devices life span and quality systems regulations may include different aspects of methods and controls in such areas as packaging, storage, labelling, manufacturing, servicing, etc. The International Organization defines the standards of international quality system for medical devices for Standardization (ISO). Moreover, the strict quality standards in production result in Good Manufacturing Practice. This is a manufacturing system with high level of standards and controls. GMP helps to reduce non-conformity, ensures greater level of products performance and safety as well as helps to maintain consistency of quality. The major benefit of quality systems is their proactive approach versus the reactive nature of “inspection-rejection” systems in manufacturing process (WHO 2003).
Other two critical elements of any quality system is documentation and people. For example, appropriate and timely documentation is a key element for any audit or inspection. And, of course, any system is only as effective as the professionals that use it. Hence, it is important to ensure that every relevant employee is aware and understand his or her input and influence to quality.
When talking about basic regulatory strategy, below Figure 2.1 reflects its major focus elements: product development and manufacture as well as market vigilance.
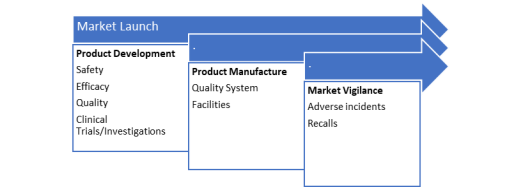
Figure 2.1. Elements of the Regulatory Strategy, adapted from (McDermott 2019).
At a product development stage, product characteristics such as safety and efficiency should be considered. The developers must generate appropriate data in order to present it for authorities review. Regulatory authorities grant authorisation for marketing and sales if the data review is satisfactory. They are also involved in approval of clinical trials and investigations that are needed for safety and effectiveness assessments. Moreover, materials, quality systems and standards can be also assessed where appropriate (Tobin 2009).
Product manufacture is another focus area of regulatory strategy. The production sites must be registered with authorities and can be inspected on a regular basis by relevant authorities. Such audits and inspections aim to verify that hygienic standards are of a certain level while efficient quality systems are in place (Tobin 2009).
The third area of focus in regulatory strategy is post-market surveillance/vigilance. It is only when the safety and performance of medical devices are continuously assessed that one can be sure that they are of the required standards. Hence, post market surveillance helps to monitor and review medical devices in use. Below Figure 2.2 shows two major activities of surveillance process:
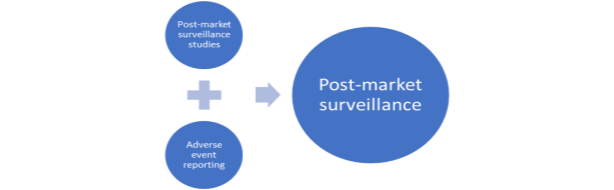
Figure 2.2 Post Market Surveillance, adapted from (WHO 2003).
Post-market surveillance studies are characterised by data collection of specific and appropriate information as condition of product approval or re-confirmation its standards when required. Adverse event reporting is characterised by registration and investigation of adverse events of medical device use that can lead to its modification/recall by authorities (WHO 2003).
References
- Europa (2019) National competent authorities, available: https://www.ema.europa.eu/en/partners-networks/eu-partners/eu-member-states/national-competent-authorities-human [accessed 15 Jan 2019].
- Europa( 2019) Notified bodies, available: https://ec.europa.eu/growth/single-market/goods/building-blocks/notified-bodies_en [accessed 15 Jan 2019].
- Europa (2019) The European regulatory system for medicines, available: https://www.ema.europa.eu/documents/leaflet/european-regulatory-system-medicines-european-medicines-agency-consistent-approach-medicines_en.pdf [accessed 15 Jan 2019].
- FDA (2018) About the FDA Organization Charts, available: https://www.fda.gov/AboutFDA/CentersOffices/OrganizationCharts/default.htm [accessed 14 Jan 2019].
- FDA (2018) About FDA Guidance Documents, available: https://www.fda.gov/RegulatoryInformation/Guidances/default.htm [accessed 13 Jan 2019].
- FDA (2017) About FDA Product Approval, available: https://www.fda.gov/NewsEvents/ProductsApprovals/ucm106288.htm [accessed 14 Jan 2019].
- FDA (2018) What does FDA regulate? https://www.fda.gov/AboutFDA/Transparency/Basics/ucm194879.htm [accessed 14 Jan 2019].
- FDA (2018) What We Do, available: https://www.fda.gov/AboutFDA/WhatWeDo/default.htm [accessed 14 Jan 2019].
- FDA (2016) Factors to Consider Regarding Benefit-Risk in Medical Device Product Availability, Compliance, and Enforcement Decisions, available: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm506679.pdf [accessed 14 Jan 2019].
- FDA (2018) Recalls, Market Withdrawals, & Safety Alerts, available: https://www.fda.gov/Safety/Recalls/default.htm [accessed 13 Jan 2019].
- HPRA (2019) About Us, available: https://www.hpra.ie/homepage/about-us [accessed 15 Jan 2019].
- IMDRF (2019) About IMDRF, available: http://www.imdrf.org/about/about.asp [accessed 1 Jan 2019].
- IMDRF (2019) International Medical Device Regulators Forum, available: http://www.imdrf.org/index.asp [accessed 15 Jan 2019].
- McDermott, O. (2019) ‘Regulatory Affairs –Introduction’, BST109: B.Sc in Science & Technology, 15 Jan, AUA, unpublished.
- Tobin J.J (2009) ‘Regulatory Compliance’, BST109, National University of Ireland, Galway, unpublished.
- WHO (2003) Medical device Regulations, Global overview and guiding principles, available: https://www.who.int/medical_devices/publications/en/MD_Regulations.pdf [accessed 13 Jan 2019].
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal



